No products in the cart.
Virus like particles (VLP) for drug delivery
Virus-like particles (VLP) Platforms for immunogens, vaccines and drug carriers
Antibody-drug Conjugate (ADC): Pre-made ADC benchmark, MOA, Production and QC
Neutralizing antibodies of virus (SARS2, HIV, HBV, Rabies, RSV, Ebola, Influenza)
Immunoglobulin Fc receptors for Fc&Fc Receptor binding assay
ILIBRA-HuEasy Monoclonal antibody (mab) humanization service (fully humanized ab)
Single domain antibody (Nanobody)
SOCAIL MEDIA

Virus like particles (VLP) for drug delivery
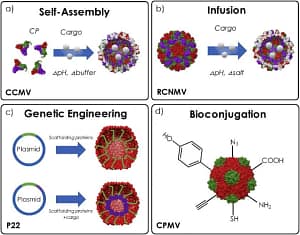
VLPs are striking agent to deliver drugs, small molecules or nucleic acids due to their biocompatibility, biodegradability and targeted delivery. Different cargo-loading techniques have been used for loading either inside or outside the capsid. Different packaging system has been shown in fig. 2.
Disassembly/reassembly of VLPs or in vitro assembly of purified proteins into VLPs. The in vitro encapsulation of small molecules/nucleic acid depends on the interactions between the capsid proteins of VLPs. Changing the buffer conditions, pH, and ionic strength in in vitro disassembles the capsid and releases the viral genome. Following disassembly, viral capsids are reassembled to encapsulate the desired molecules using buffer exchange methods. VLPs are porous and the materials “breeze” inside and change shape under various conditions to permit the infusion of small molecules into the capsid’s cavity. This technique involves the addition of chelators to remove calcium and magnesium ions from the solvent leading to the formation of 11–13 Å-diameter channels to infuse the small molecules, later, the channels are closed by the addition of ion.
Genetic engineering based in vivo assembly is another technique for the encapsulation of peptides into VLPs. It involves the insertion of peptide encoding gene sequences into the genes encoding the capsid protein. The peptide and capsid protein can be expressed simultaneously or separately and it self-assemble through either in vivo or in vitro.
Besides encapsulation techniques, small molecules can also covalently attach to the VLPs with use of reactive amino acid side chains of the protein structure. Functional addressable groups are principally amines (Lys), carboxylates (Asp, Glu), thiols (Cys), and aromatic groups (Tyr, Trp) on the capsid are utilized for conjugation.
View the Knowledge base of virus like particles (VLP):
– Virus-like particles (VLP) Platforms for Developing immunogens, vaccines and drug carriers
– What is virus like particles (VLP)?
– GM VLPxTM Virus-like particles development platform
– Virus like particles (VLP) Expression platforms
– Virus like particles (VLP) as immunogens/antigens of transmembrane protein (TM)
– Virus like particles (VLP) for drug delivery
– Virus like particles (VLP) as vaccine
– Virus like particles (VLP) for drug discovery