Adenovirus vector system (adenovirus expression system, adenovirus packaging plasmid system)
Introduction to Adenovirus vector system
GeneMedi’s adenovirus Vector System, also named the adenovirus expression system or adenovirus packaging plasmid system, is a powerful tool for in-vitro & in-vivo gene delivery, shRNA mediated RNA interference (RNAi) and gene editing. You can easily recombine produce a recombinant adenovirus (r-AdV) particle in HEK293 or HEK293A cell line in high titer using GeneMedi’s adenovirus Vector System.
The Genemedi adenovirus vector system including 2 types of adenovirus packaging plasmids: the adenovirus expression plasmids (adenovirus shuttle vectors) with different promoters and tags, and an adenovirus genome backbone plasmid with E1 region deletion.
Note:
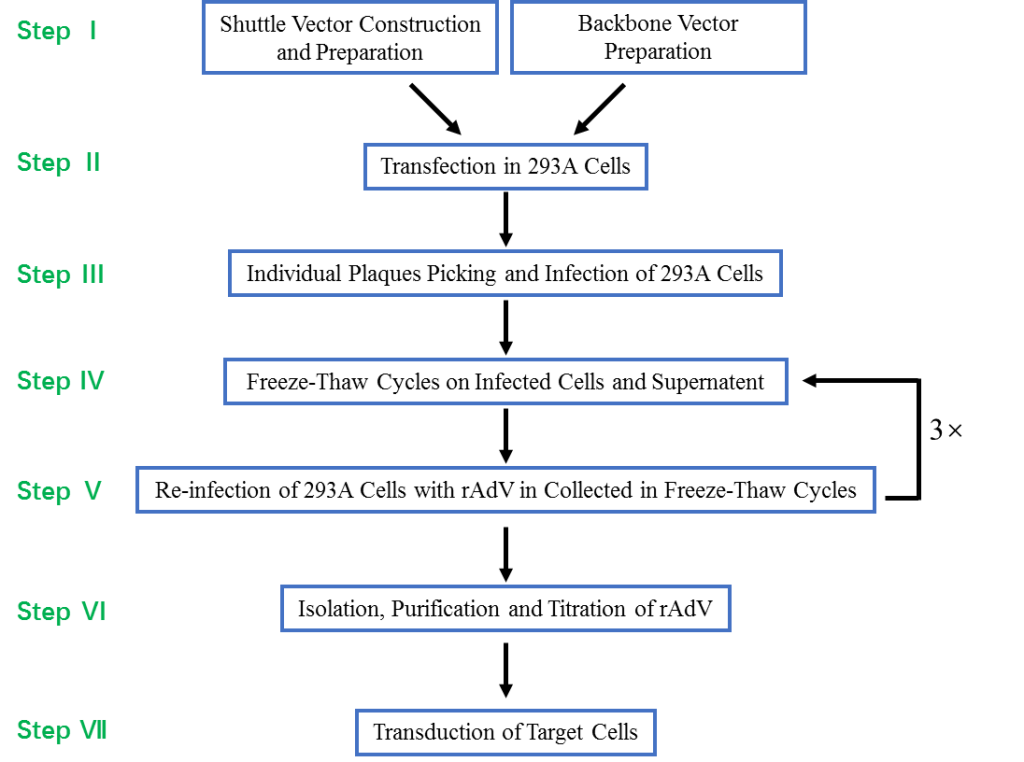
Shuttle Vector Construction and Preparation Backbone Vector Preparation
Transfection in 293A Cells
Individual Plaques Picking and Infection of 293A Cells
Freeze-Thaw Cycles on Infected Cells and Supernatent
Re-infection of 293A Cells with rAdV in Collected in Freeze-Thaw Cycles
Isolation, Purification and Titration of rAdV
Transduction of Target Cells
GeneMedi has developed a variety of adenovirus expression vectors with different expression cassettes, containing kinds of verified promoters and reporters including GFP, zsgreen, RFP, mcherry and luciferase. The GeneMedi’s adenovirus expression vectors have been proved very suitalble for unique gene overexpression or shRNA-mediated knock-down (also called RNAi (RNA interference ). You can also achieve gene knock-out(KO) or gene editing using our Crispr-cas9-gRNA adenovirus expression vector.
Introduction to Adenovirus Vector System (Adenovirus packaging and expression system)
Finish adenovirus packaging in 2 weeks with Genemedi’s FasterAd Adenovirus vector system (adenovirus packaging system). The Genemedi’s FasterAd Adenovirus packaging system using a HEK293 recombination system including the adenovirus expression vector (adenovirus overexpression or shRNA vector) and the adenovirus packaging plasmid (adenovirus backbone).
Recombinant adenovirus (Adenovirus), a replication-defective adenoviral vector system, is widely used for gene delivery in most cell types. The adenoviral vectors provided by Genemedi are based on human adenovirus type 5 (Ad5), which is replication-incompetent (-E1/-E3) and can’t be integrated into host genome, guaranteeing the security for subsequent operations. Adenovirus packaging by Genemedi shows almost 100% gene delivery in most cell types in the recombinant protein expression system both in vivo and in vitro. Recombinant protein expression of interest is detectable from 24 hours post infection.
An adenovirus packaging system including the adenovirus expression vector and the adenovirus package plasmid. In order to meet different infection needs, Genemedi has launched a variety of adenovirus packaging system plasmids with different fluorescent labels (such as GFP, RFP, Luciferase and so on), and various promoters or protein tags, which may also allow researchers to produce lentiviruses by themselves as they need.
Adenovirus vector system
Adenovirus expression vectors (adenovirus expression plasmids)
Note: Industry R&D excludes CRO&CDMO&CXO; Others includes CRO&CDMO&CXO&CMC&Manufacturing company
Adenovirus genome backbone plasmid
Advantages
1. Broad range of host. Adenovirus has the ability to infect dividing, quiescent cells, stem cells, and primary cells, allowing genetic materials to be delivered to a highly diverse range of cell types and tissues.
2. High infection efficiency. Almost 100% gene delivery in most cell types, completely surpassing other viral vector tools and liposome transfection.
3. Great packaging capacity, up to 8kb.
4. Without integration. No alteration to host genome.
5. High titer. Up to 1011 PFU/ml.
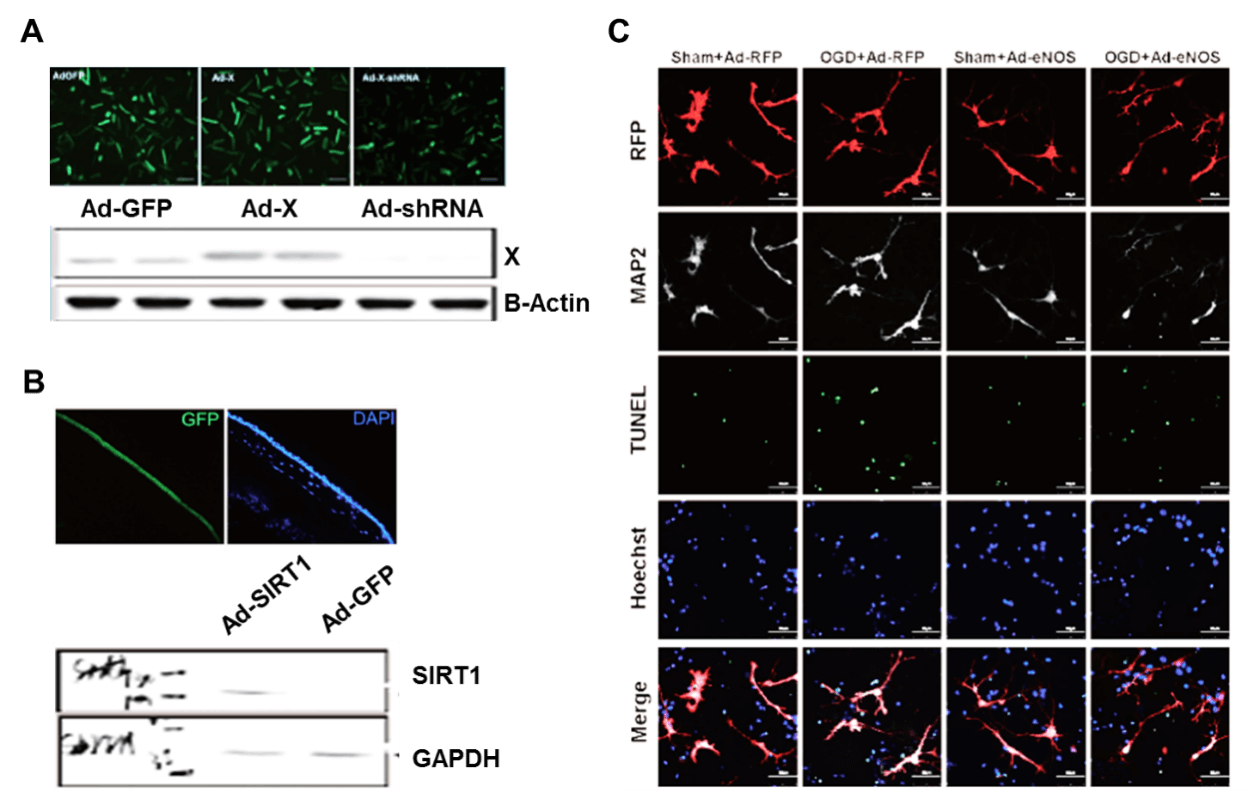
Applications and Figures
Technical Documents
 Adenovirus User Manual.
Adenovirus User Manual. Reference
1.Hu J, L Zhang, Y Yang, Y Guo, Y Fan, M Zhang, W Man, E Gao, W Hu, RJ Reiter, H Wang and D Sun. (2017). Melatonin alleviates postinfarction cardiac remodeling and dysfunction by inhibiting Mst1. J Pineal Res 62.
2.Xu C, F Wu, C Mao, X Wang, T Zheng, L Bu, X Mou, Y Zhou, G Yuan, S Wang and Y Xiao. (2016). Excess iodine promotes apoptosis of thyroid follicular epithelial cells by inducing autophagy suppression and is associated with Hashimoto thyroiditis disease. J Autoimmun 75:50-57.
3.Wang Y, X Zhao, X Wu, Y Dai, P Chen and L Xie. (2016). microRNA-182 Mediates Sirt1-Induced Diabetic Corneal Nerve Regeneration. Diabetes 65:2020-31.
4.Luo T, J Fu, A Xu, B Su, Y Ren, N Li, J Zhu, X Zhao, R Dai, J Cao, B Wang, W Qin, J Jiang, J Li, M Wu, G Feng, Y Chen and H Wang. (2016). PSMD10/gankyrin induces autophagy to promote tumor progression through cytoplasmic interaction with ATG7 and nuclear transactivation of ATG7 expression. Autophagy 12:1355-71.
5.Liu TY, XQ Xiong, XS Ren, MX Zhao, CX Shi, JJ Wang, YB Zhou, F Zhang, Y Han, XY Gao, Q Chen, YH Li, YM Kang and GQ Zhu. (2016). FNDC5 Alleviates Hepatosteatosis by Restoring AMPK/mTOR-Mediated Autophagy, Fatty Acid Oxidation, and Lipogenesis in Mice. Diabetes 65:3262-3275.
6.Liu H, S Fang, W Wang, Y Cheng, Y Zhang, H Liao, H Yao and J Chao. (2016). Macrophage-derived MCPIP1 mediates silica-induced pulmonary fibrosis via autophagy. Part Fibre Toxicol 13:55.
7.Yao T, X Ying, Y Zhao, A Yuan, Q He, H Tong, S Ding, J Liu, X Peng, E Gao, J Pu and B He. (2015). Vitamin D receptor activation protects against myocardial reperfusion injury through inhibition of apoptosis and modulation of autophagy. Antioxid Redox Signal 22:633-50.
8.Wang B, A Ma, L Zhang, WL Jin, Y Qian, G Xu, B Qiu, Z Yang, Y Liu, Q Xia and Y Liu. (2015). POH1 deubiquitylates and stabilizes E2F1 to promote tumour formation. Nat Commun 6:8704.
9.Hua F, K Li, JJ Yu, XX Lv, J Yan, XW Zhang, W Sun, H Lin, S Shang, F Wang, B Cui, R Mu, B Huang, JD Jiang and ZW Hu. (2015). TRB3 links insulin/IGF to tumour promotion by interacting with p62 and impeding autophagic/proteasomal degradations. Nat Commun 6:7951.
10.Wang X, J Liu, J Zhen, C Zhang, Q Wan, G Liu, X Wei, Y Zhang, Z Wang, H Han, H Xu, C Bao, Z Song, X Zhang, N Li and F Yi. (2014). Histone deacetylase 4 selectively contributes to podocyte injury in diabetic nephropathy. Kidney Int 86:712-25.




