No products in the cart.
Adenovirus-LC3 production service for Autophagy Flux Detection
Customized ADV production
Introduction to AAV-LC3 production service for Autophagy Flux Detection
Premade LC3 Autophagy Biosensors Products and user manual
|
Adeno associated virus(AAV) AAV-GFP-LC3 Autophagy Biosensor AAV-mRFP-GFP-LC3 Autophagy Biosensor |
Adenovirus Adv-GFP-LC3 Autophagy Biosensor Adv-mRFP-GFP-LC3 Autophagy Biosensor |
Lentivirus Lv-GFP-LC3 Autophagy Biosensor Lv-mRFP-GFP-LC3 Autophagy Biosensor |
LC3 Autophagy Biosensors User Manual
Autophagy is a highly regulated homeostatic degradative process where cells destroy their own components via the lysosomal machinery and recycle them. This process plays both protective and deleterious roles in many diseases, including Alzheimer’s disease, aging, cancer, infection and Crohn’s disease. Elucidating the correlation between autophagy and apoptotic cell death has become the focus of a great deal of research. Members of the LC3 family play a key role in the maturation of the autophagosome. Lysosomal turnover of the autophagosomal marker LC3-II reflects starvation-induced autophagic activity, and detecting LC3 by immunoblotting or immunofluorescence has become a reliable method for monitoring autophagy and autophagy-related processes, including autophagic cell death.
Genemedi has launched series of Adenovirus packaging service of autophage related biosensors, in which GFP and/or RFP tags are fused at the C-termini of the autophagosome marker LC3, allowing to detect the intensity of autophagy flux in real-time with more accuracy, clarity and intuitiveness. These biosensors provides an enhanced dissection of the maturation of the autophagosome to the autolysosome, which capitalizes on the pH difference between the acidic autolysosome and the neutral autophagosome. The acid-sensitive GFP will be degraded in autolysosome whereas the acid-insensitive RFP will not. Therefore, the change from autophagosome to autolysosome can be visualized by imaging the specific loss of the GFP fluorescence, leaving only red fluorescence. Taking advantage of RFP-GFP-LC3 and GFP-LC3 labeling system, Genemedi has launched the production service of Adv-RFP-GFP-LC3 and Adv-GFP-LC3, which can be used to observe autophagy flux and monitor the intensity of autophagy flux in real-time in vivo or in vitro.
Properties
| Adenovirus Vector | |
|---|---|
| Quantity/Unit | Vials |
| Form | Frozen form |
| Suitable Types of Infection | In vivo infection in animals |
| Sipping and Storage Guidelines | Shipped by dry ice, stored at -80 ℃, effective for 1 year. Avoid repeatedly freezing and thawing. |
| Titer | > 1*10^10 PFU/ml |
Advantages
1. Autophagy flux detection in real time, quantitative.
2. High resolution, more accuracy and sensitivity than traditional approaches.
3. Broad range of host. Adv has the ability to infect dividing, quiescent cells, stem cells, and primary cells, allowing genetic materials to be delivered to a highly diverse range of cell types and tissues.
4. High infection efficiency. Almost 100% gene delivery in most cell types, completely surpassing other viral vector tools and liposome transfection.
5. Great packaging capacity, up to 8kb.
6. Without integration. No alteration to host genome.
7. High titer. Up to 1011 pfu/ml.
Applications and Figures
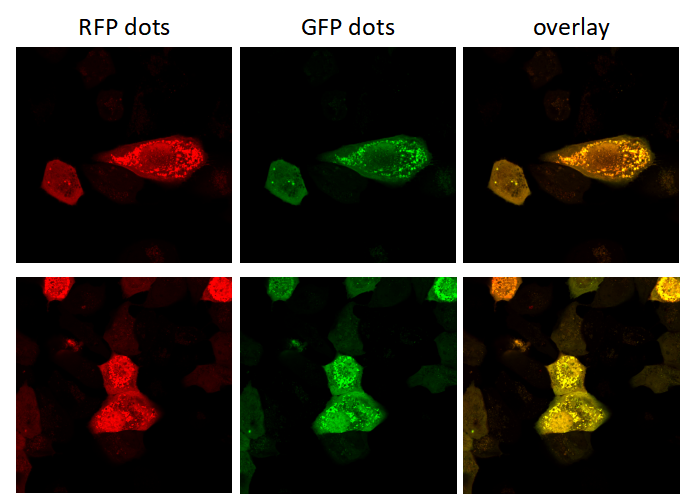
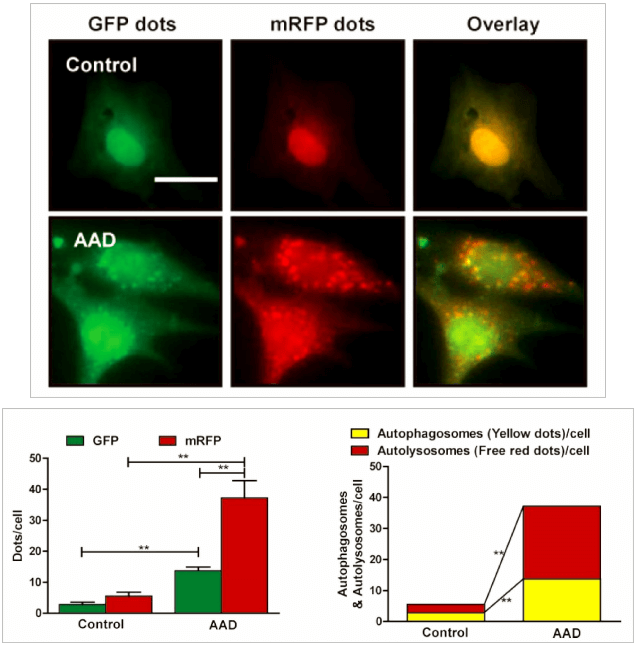
Quality control description
Our optimized custom adenoviral vector production and strict quality control systems provide customers a high titer of functional recombinant adenoviral vectors. Viral titers are determined by TCID50 method, which is the most accurate way to measure the titer of adenovirus.
Technical Documents
1. For detailed protocol about how to package and purify Adv, please see the  Adenovirus User Manual on the Genemedi website.
Adenovirus User Manual on the Genemedi website.
2. For further information about Adv autophagy flux detection system, please refer to the  Adenovirus Autophagy Flux Detection Manual on the Genemedi website.
Adenovirus Autophagy Flux Detection Manual on the Genemedi website.
Reference
1.Hariharan N, P Zhai and J Sadoshima. (2011). Oxidative stress stimulates autophagic flux during ischemia/reperfusion. Antioxid Redox Signal 14:2179-90.
2.Yuan Y, Y Zheng, X Zhang, Y Chen, X Wu, J Wu, Z Shen, L Jiang, L Wang, W Yang, J Luo, Z Qin, W Hu and Z Chen. (2017). BNIP3L/NIX-mediated mitophagy protects against ischemic brain injury independent of PARK2. Autophagy 13:1754-1766.
3.Yu T, F Guo, Y Yu, T Sun, D Ma, J Han, Y Qian, I Kryczek, D Sun, N Nagarsheth, Y Chen, H Chen, J Hong, W Zou and JY Fang. (2017). Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 170:548-563 e16.
4.Shao BZ, P Ke, ZQ Xu, W Wei, MH Cheng, BZ Han, XW Chen, DF Su and C Liu. (2017). Autophagy Plays an Important Role in Anti-inflammatory Mechanisms Stimulated by Alpha7 Nicotinic Acetylcholine Receptor. Front Immunol 8:553.
5.Liu M, K Liang, J Zhen, M Zhou, X Wang, Z Wang, X Wei, Y Zhang, Y Sun, Z Zhou, H Su, C Zhang, N Li, C Gao, J Peng and F Yi. (2017). Sirt6 deficiency exacerbates podocyte injury and proteinuria through targeting Notch signaling. Nat Commun 8:413.
6.Chen ZH, WT Wang, W Huang, K Fang, YM Sun, SR Liu, XQ Luo and YQ Chen. (2017). The lncRNA HOTAIRM1 regulates the degradation of PML-RARA oncoprotein and myeloid cell differentiation by enhancing the autophagy pathway. Cell Death Differ 24:212-224.
7.Zhang Y, K Shen, Y Bai, X Lv, R Huang, W Zhang, J Chao, LK Nguyen, J Hua, G Gan, G Hu and H Yao. (2016). Mir143-BBC3 cascade reduces microglial survival via interplay between apoptosis and autophagy: Implications for methamphetamine-mediated neurotoxicity. Autophagy 12:1538-59.
8.Luo T, J Fu, A Xu, B Su, Y Ren, N Li, J Zhu, X Zhao, R Dai, J Cao, B Wang, W Qin, J Jiang, J Li, M Wu, G Feng, Y Chen and H Wang. (2016). PSMD10/gankyrin induces autophagy to promote tumor progression through cytoplasmic interaction with ATG7 and nuclear transactivation of ATG7
9.Hua F, K Li, JJ Yu, XX Lv, J Yan, XW Zhang, W Sun, H Lin, S Shang, F Wang, B Cui, R Mu, B Huang, JD Jiang and ZW Hu. (2015). TRB3 links insulin/IGF to tumour promotion by interacting with p62 and impeding autophagic/proteasomal degradations. Nat Commun 6:7951.
10.Zhang X, Y Yuan, L Jiang, J Zhang, J Gao, Z Shen, Y Zheng, T Deng, H Yan, W Li, WW Hou, J Lu, Y Shen, H Dai, WW Hu, Z Zhang and Z Chen. (2014). Endoplasmic reticulum stress induced by tunicamycin and thapsigargin protects against transient ischemic brain injury: Involvement of PARK2-dependent mitophagy. Autophagy 10:1801-13.




