TCR-T immunotherapy and cancer therapy
Cell therapy is a kind of medicine aiming to cure disease or alleviate disease symptoms via direct infusion or transplantation of cells, which can be autologous or allogeneic. With several decades’ development and optimization, immuno-oncology cells (such as T cells, nature killer cells, etc.), stem cells (embryonic stem cells, induced pluripotent stem cells, progenitor cells, etc.) or other genetic re-engineered cells have been widely applied for cell therapy. Numerous cell types have been translated into clinical trials and promising cell therapy outcomes have been achieved from Phase I, Phase II and Phase III trials for a great number of diseases.
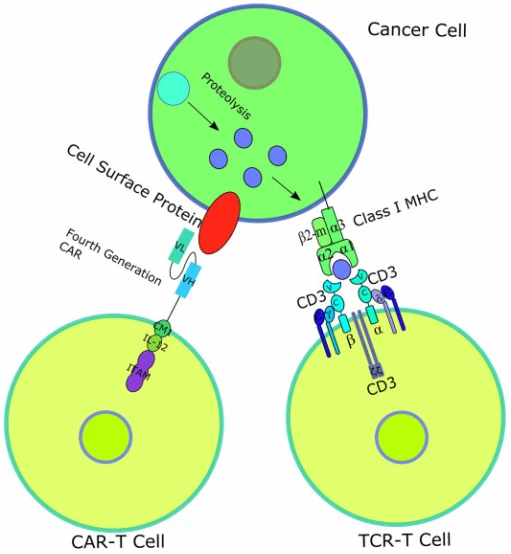
T-cell receptor (TCR)–engineered T-cell therapy (TCR-T) is one potentially powerful treatment that utilizes genetically modified natural T cells to specifically target tumors and destroy tumors with greater potentials [37]. Unlike CARs, TCRs rely on their interactions with peptide-major histocompatibility complex (pMHC), formed by peptide [38,39], generated from intracellular antigen proteolysis, bound with MHC (Fig. 3) [40]. Besides TCR, additional costimulatory or co-inhibitory signals are also required for TCR-T function. For example, CD4 on the surface of helper T cells, which binds to class II MHC complex, and CD8 on the surface of cytotoxic T cells, which binds to class I MHC complex, activates the TCR-T mediated cell destruction [41], while cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1) are responsible for T cell signaling extinguisher [42]. Given that only ~28% antigens are expressed on the cell surface, it is difficult for most of the antigens to be recognized by CAR [43]. Therefore, compared to CAR-T, TCR-T cell therapy shows remarkable advantages over the destruction of tumor cells with intracellular antigens [44]. To date, TCR-T mediated cell therapy has achieved many promising curative potency in both solid tumors and hematological cancers, and some of them have been applied in clinic trials (Table 2).
| Antigen | Disease | Phase of clinical trials | NCT/Reference |
| PRAME | Unknown | Phase I/II | NCT03503968 |
| PRAME | Acute myeloid leukemia, myelodysplastic syndrome | Phase I | NCT02743611 |
| MAGE-A3/A6 | Unknown | Phase I | NCT03139370 |
| Unknown | Head and neck squamous cell carcinoma, squamous cell NSCLC | Phase I | NCT03139370 |
| MAGE-A10 | Non-small cell lung carcinoma | Phase I | NCT02592577 |
| NY-ESO-1 | Synovial sarcoma | Phase I/II | NCT01343043 |
| NY-ESO-1 | Multiple myeloma | Phase I/II | NCT01892293 |
| NY-ESO-1 | Multiple myeloma | Phase I/II | NCT01352286 |
| NY-ESO-1 | Ovarian cancer | Phase I/II | NCT01567891 |
| NY-ESO-1 | Melanoma | Phase I/II | NCT01350401 |
| NY-ESO-1 | Non-small cell lung carcinoma | Phase I/II | NCT02588612 |
| AFP | Hepatocellular cancer | Phase I | NCT03132792 |
| NY-ESO-1 | Refractory multiple myeloma | Phase I | NCT03168438 |
| MAGE-A10 | Urinary bladder cancer, head and neck cancer, melanoma | Phase I | NCT02989064 |
CAR-T vs TCR-T
CAR-T and TCR-T both are gene-engineered technologies targeted for the destruction of tumors by improving the recognition and destroy potentials of T cells, thus called “T cell receptor redirection” technologies. However, there are also some differences in the two technologies: 1) CAR-T recognizes their target antigen via the scFv binding domain of CAR, while TCR-T relies on the interactions with peptide-major histocompatibility complex (pMHC) and requires co-stimulatory od co-inhibitory signals; 2) CAR-T recognizes and targets cell surface antigens, while TCR-T can target both cell surface antigens and intracellular antigens; 3) CAR-T shows great therapy outcomes in the treatment of hematological cancers but little effects on solid tumors, while TCR-T displays encouraging curative outcomes in the therapy of solid tumors.




