Lentivirus vector system, lentivirus production and transduction
Lentivirus vector -Introduction
Landscape, protocol and guidelines of lentivirus vector system, lentivirus production, lentivirus transduction and lentivirus gene therapy are described below. The detail information is mentioned about lentivirus plasmid cloning, lentivirus packaging, purification,lentivirus-mediated stable cell lines development and the insight of lentivirus gene therapy.
Lentivirus vector -Index
Lentivirus vector and stable cell line development-Knowledge Base
1. What is Lentivirus?
Lentivirus (lente-, Latin for “slow”) is a genus of retroviruses, causing chronic and deadly diseases by long incubation periods in human or other mammalian species [1]. The virion is a medium-sized (80-100nm) and enveloped, slightly pleomorphic, spherical with an isometric nucleocapsid containing two copies of positive-sense ssRNA genome. Most lentiviral vectors are based on the Human Immunodeficiency Virus (HIV), which causes AIDS. To date, 5 serogroups have been recognized according to the vertebrate hosts they are associated with (primates, sheep and goats, horses, domestic cats, and cattle). Among them, the primate lentiviruses are distinguished by the utilization of CD4 surface protein as a receptor and the absence of dUTPase [2]. Considering the key safety concerns during the use of HIV-derived lentivirus vectors, recombinant lentivirus has been designed and widely used for gene delivery in most cell types.
Derived from HIV-1, lentiviruses can integrate a significant amount of viral cDNA into the host genome, mediate stable and long-term transgene expression, and efficiently infect dividing cells and nondividing cells, which makes lentivirus an attractive gene delivery vehicles in most cell types [3].
| Indication | Transplants | Delivery route | Phase | Sponsor |
| β-Thalassemia | Autologous CD34+ cells transduced with Lenti-TNS9.3.55 | Intravenous | I | Memorial Sloan Kettering Cancer Center |
| Autologous CD34+ cells transduced with LentiGlobin BB305 | Intravenous | Ⅲ | bluebird bio | |
| Autologous CD34+ cells transduced with LentiGlobin BB305 | Intravenous | I–II | bluebird bio | |
| Autologous CD34+ cells transduced with Lenti- β-globin | Intravenous | I–II | IRCCS San Raffaele | |
| X-linked adrenoleukodystrophy | Lenti-ABCD1 | Intracerebral | I–II | Shenzhen Geno-Immune Medical Institute |
| Autologous CD34+ cells transduced with Lenti-ABCD1 | Intravenous | I–II | Shenzhen Second People’s Hospital | |
| Wiskott-Aldrich Syndrome | Autologous CD34+ cells transduced with Lenti-WAS gene | Intravenous | I–II | Genethon |
| Autologous CD34+ cells transduced with Lenti-WAS gene | Intravenous | II | GlaxoSmithKline | |
| Autologous CD34+ cells transduced with Lenti-WAS gene | Intravenous | I–II | Genethon | |
| Autologous CD34+ cells transduced with Lenti-WAS gene | Intravenous | I–II | Genethon | |
| Metachromatic leukodystrophy | Lenti-ARSA | intracerebral | Not applicable | Not applicable |
| Autologous CD34+ stem cells transduced with Lenti-ARSA | Intravenous | II | GlaxoSmithKline | |
| Autologous CD34+ stem cells transduced with Lenti-ARSA | Intravenous | I–II | Shenzhen Second People’s Hospital | |
| Sickle cell disease | Lenti-gamma-globin | Intravenous | I–II | Children’s Hospital Medical Center, Cincinnati |
| Autologous CD34+ stem cells transduced with Lenti-βAS3-FB | Intravenous | I | Donald B. Kohn, M.D. | |
| Autologous CD34+ cells transduced with LentiGlobin BB305 | Intravenous | I | Bluebird bio | |
| Autologous CD34+ cells transduced with Lenti-shBCL11a | Intravenous | I | David Williams | |
| Fanconi anemia | Autologous CD34+ cells transduced with Lenti- FANCA | Intravenous | I | Fred Hutchinson Cancer Research Center |
2. Lentivirus Gene Therapy
Lentivirus has been proved as an excellent gene therapy vector. To date, more than 236 clinical trials have been carried out using lentivirus vectors for gene delivery [4], and promising gene therapy outcomes from recombinant lentivirus have been achieved from clinical trials for a great number of diseases (Table 1), especially for cancer treatment. As a research tool used to introduce a gene product into in vitro systems or animal models, lentiviral vector has been put into large-scale efforts to down-regulate or up-regulate gene expression in high-throughput formats, allowing researchers to examine the necessity and effects of transgenes in disease model systems, which is an indispensable for the discovery of novel transgenic drugs. To date, lentiviral vector-based gene delivery into CD34+ HSCs has been used as an alternative in clinical trials and proved to be effective in treatment of several diseases [5], including β-thalassemia [6], X-linked adrenoleukodystrophy (ALD) [7], metachromatic leukodystrophy [8,9], and Wiskott-Aldrich Syndrome [10]. Among them, HSCs transduced with β-globin in patients with β-thalassemia, one patient with βE/β0-thalassemia achieved independence from transfusion [6]; HSCs transduced with wild-type ABCD1 in two patients with X-linked adrenoleukodystrophy (ALD), they achieved polyclonal reconstitution, with 9 to 14% of granulocytes, monocytes, and T and B lymphocytes expressing the ALD protein with the stop of progressive cerebral demyelination [7]; Functional WASP transduced by lentiviral vector into three WAS patients to genetically correct HSPCs, all the three patients showed improvements in platelet counts, immune functions, and clinical scores [10]. No adverse events related to the vector have been reported in these clinical trials.
3. Advantages and Drawbacks of Lentivirus Vector-mediated gene delivery
a) Advantages of lentivirus -mediated gene delivery
Lentivirus has been developed as an attractive candidate for creating viral vectors for gene therapy due to various advantages.
1) Customized cloning for any other gene ORF expression, shRNA/miRNA and CRISPR/Cas9.
2) No known immunogenic proteins generated.
3) High titer. 108TU/ml or 109TU/ml lentiviral titer for cell line transfection in medium or large scale.
4) With broad range of hosts. Mediate efficient transfection in both dividing and non-dividing cells.
5) Integration into host cell genome, mediating long-term and stable expression of exogenous genes.
6) Deliver complex genetic elements, such as intron-containing sequences.
7) Simple system for vector manipulation and production.
b) Drawbacks of lentivirus-mediated gene transfer
Although lentivirus benefits a great deal of disease therapies, it does present some drawbacks.
1) Based on HIV-1, recombinant lentivirus vectors require at least three HIV-1 genes (gag, pol, and rev) for production, which is still not safe enough for gene therapy. To date, the best solution for this drawback is to turn to adenovirus or AAV vectors, which may be safer than lentivirus vector.
| Comparison | Retrovirus | Lentivirus | Adenovirus | AAV |
| Genome | ss RNA | ss RNA | ds DNA | ss DNA |
| Integration | Yes | Yes | no | no |
| Packaging Capacity | 3kb | 4kb | 5.5kb | 2kb |
| Time to peak expression | 72h | 72h | 36h-72h | cell: 7 days; animals: 2 weeks |
| Sustainable time | about 3 weeks | stable expression | transient expression | > 6 months |
| Cell Type | most dividing/non-dividing Cells | most dividing/non-dividing Cells | most dividing/non-dividing Cells | most dividing/non-dividing Cells |
| Titer | 10^7 TU/ml | 10^8 TU/ml | 10^11 PFU/ml | 10^12 vg/ml |
| Animal experiment | suitable | low efficiency | lowest efficiency | most suitable |
| Immune Response | high | medium | medium | mild |
4. Lentivirus Genome Structure and Virus Assembly
Lentivirus, as represented by HIV-1, is a medium-sized (80-100nm) and enveloped, slightly pleomorphic, spherical virus with an isometric nucleocapsid (Figure1A). Unlike other retroviruses, HIV-1 is featured by a set of additional regulatory and accessory genes [11,12]. Its DNA genome, transcribed from HIV-1 ssRNA, is approximately 9.7 kb and contains 9 ORFs. Besides gag, pol, and env (typical of all retroviruses), there are two regulatory (tat and rev) and four accessory (vif, vpr, vpu, and nef) genes specific for HIV-1 (Table 3) as well. All of these protein-coding regions are flanked by 5’ and 3’ LTRs (LTR, long terminal repeat) which are required for HIV-1 life cycle, such as reverse transcription, integration, and gene expression. HIV-1 gene transcription is a complex process, characterized by its specific pattern of viral gene regulation [13]. Several cis-acting sequences, harbored by the HIV-1 LTR, is required for the initiation of viral RNA expression. More than 30 kinds of RNA are converted from the integrated HIV-1 DNA, falling into three classes mRNA according to the splicing pattern, i.e. unspliced, partially spliced, and multiply spliced RNAs [13-15]. The unspliced transcript (about 9kb) is full-length RNA which needs to be packaged as the viral genome into new viral particle but it also functions as mRNA to produce gag and gag-pol polyproteins. The partially spliced transcripts (about 4kb) encode accessory proteins vif, vpr, vpu, and env structural proteins. The multiply spliced RNAs are predominant following the virus infection, and their encoded proteins, the regulatory proteins tat, rev, and nef are highly produced to increase virus infectivity and regulate transcription [16].

| Classification | Gene | Encoding proteins and functions |
| Structural proteins | env | gp120, surface envelope protein SU; gp41, transmembrane envelope protein TM |
| gag | p24, capsid protein CA; p17, matrix protein MA; p9, capsid protein NC | |
| Enzymes | pol | Reverse transcriptase RT, 66kD; Integrase IN, 32kD |
| pro | Protease PR; dUTPase DU | |
| Gene regulatory proteins | tat | HIV transactivator, positive regulator of transcription |
| rev | Regulator of virion proteins expression, essential for major viral proteins synthesis and viral replication | |
| Accessory proteins | vif | Viral infectivity, required for infectivity in some cell types |
| vpr | Virus protein R, nuclear import of pre-integration complex and host cell cycle arrest | |
| vpu | Virus protein U, proteasomal degradation of CD44 and release of virions from infected cells | |
| nef | Negative factor, role in apoptosis and key in increasing virus infectivity |
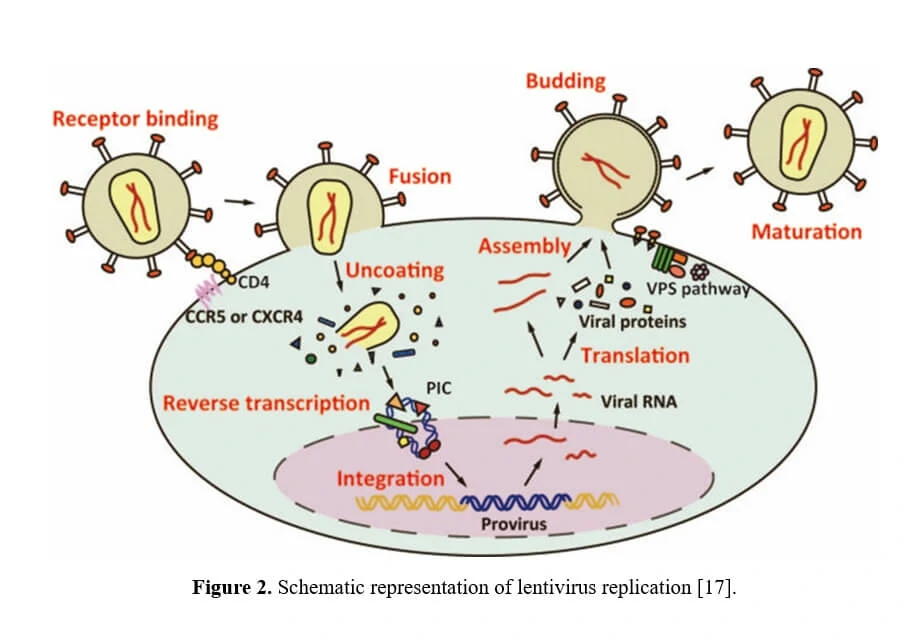
5. Life Cycle of Lentivirus
The life cycle of HIV-1 starts with viral entry, in which process the virus binds to the CD4 receptor or a coreceptor (CCR5 or CXCR4) with gp120 protein, thereby anchoring itself onto host cell surface, allowing fusion between the cellular and viral membranes. After entry into the cell, the viral nucleoprotein together with the contents, i.e. the genomic ssRNA, is released into cytoplasm. Then, utilizing the cellular nucleotides as the building blocks, double-stranded DNA is generated from the virus genome ssRNA directed by the HIV-1 reverse transcriptase (RT) in a nucleoprotein complex termed the RTC. Together with other viral proteins, the newly synthesized DNA constitutes an integration-competent nucleoprotein complex, migrating into host cell nucleus and mediating integration of viral DNA into host chromatin. Integrated viral DNA, named as provirus, becomes part of host genome and serves as a transcription template for the synthesis of viral mRNA and genomic RNA. Following the synthesis of viral genomes and proteins (Table 3), the viral components are assembled together to produce new virions, the virus particles then bud out of host cell and undergo a maturation step to generate infectious HIV-1.
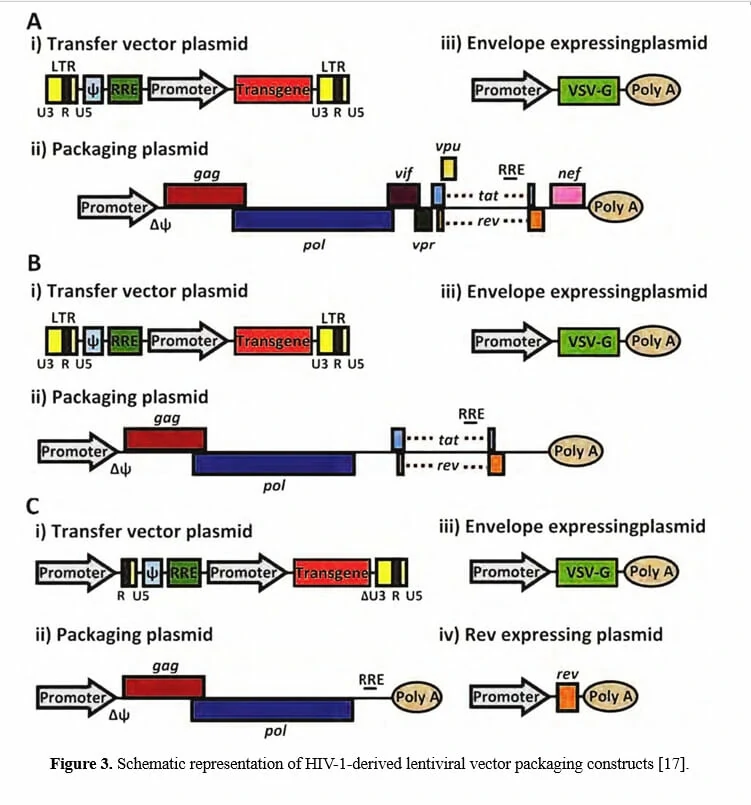
Considering that HIV-1 is a human pathogen which destructs CD4+ helper T lymphocytes and results in the subsequent loss of immune competence, in order to guarantee the experiments safety, efficient HIV-1-derived lentiviral vectors have been developed with improved biosafety features and regarded as a safe gene delivery tool to replace wild-type HIV-1 (Figure 3) [19].
6. Recombinant lentivirus
a) Introduction of recombinant lentivirus vector system
Since wild-type HIV-1 based lentivirus is associated with destruction of host immune system, especially CD4+ helper T lymphocytes, multiple generations of lentivirus vectors have been designed with enhanced safety features and as attractive vectors for gene therapy. To date, there have been three generations of lentivirus vectors (the designing schemes are shown in Figure 3), differing in the extent to which the genome from wild-type HIV-1 is attenuated. The first-generation is three plasmids co-transfection in 293T cells, including all HIV genes with the exception of the env gene in another plasmid, encoding vesicular stomatitis virus G protein (VSV-G) which may improve the stability and broaden the cellular tropism of lentiviral vectors [20,21]. Due to the potential risk for the generation of replication competent lentiviruses (RCL) and of the recombination event during subsequent reverse transcription in transduced cells, researchers have further developed the second-generation lentivirus vectors by eliminating all accessory proteins (vif, vpr, vpu, and nef) via mutation or deletion of these genes from the packaging plasmid, overcoming the safety issue attributable to the first-generation vectors [22-24]. To avoid the homologous recombination between the vector genome and wild-type HIV-1 in case that the lentiviral vectors are used for gene therapy of HIV/AIDS, the third-generation lentivirus vectors have been designed by modifying the 3’ LTR, deleting tat, and providing rev in a separate plasmid, offering the best safety profile in terms of generation of RCL [25-27].
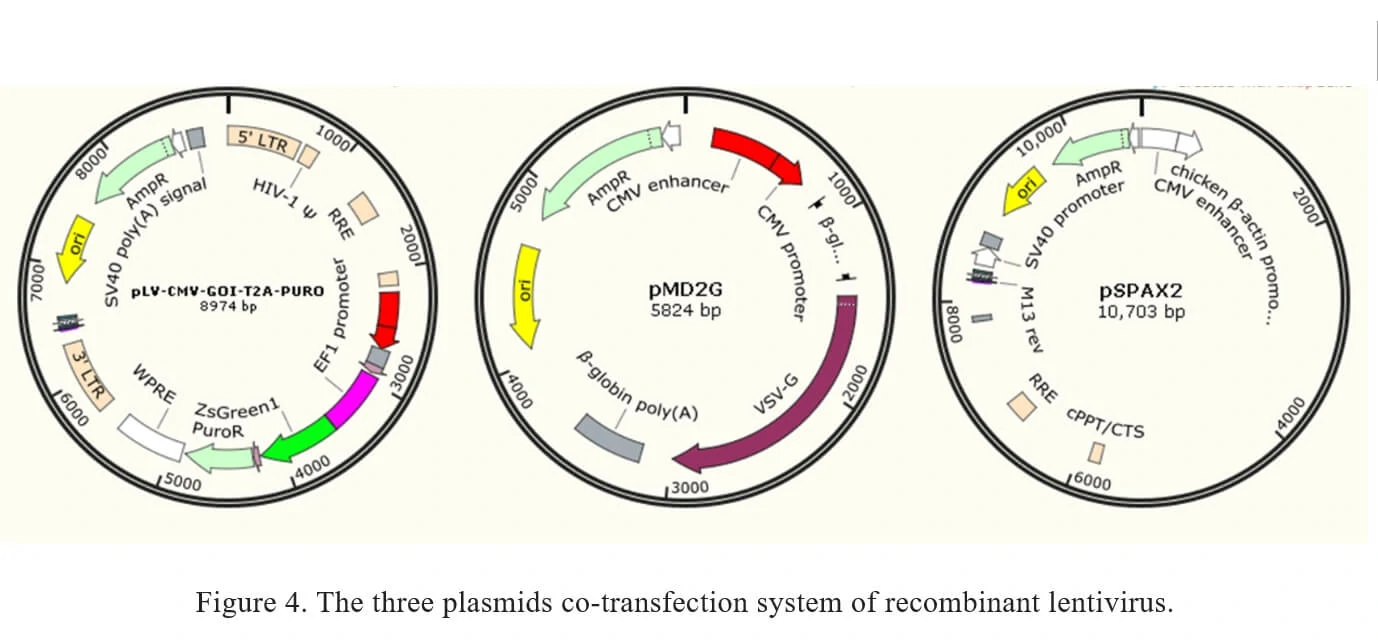
Traditionally, recombinant lentivirus vectors used in Genemedi were prepared with a plasmid containing the transgene flanked by long terminal repeats (LTRs), co-transfected with envelope expressing plasmid pMD2G and packaging plasmid pSPAX2. Once packaged into 293T, recombinant lentiviral vectors will be easily propagated.
b) Recombinant lentivirus system
The current method of the recombinant lentivirus production in Genemedi is based on three plasmids co-transfection system. It involves the co-transfection of 3 plasmids into 293T cells as shown in Figure 4.
1. pLV-CMV-MCS-T2A-PURO: a lentivirus LTR-containing plasmid carrying multiple clone sites, which can be cloned into a transgene.
2. pMD2G: envelope expressing plasmid.
3. pSPAX2: packaging plasmid.
7. Lentivirus Infection Protocol for stable cell line development (CLD)
This protocol is for the stable cell line construction based on puromycin selection.
Day 1: Seed target cells in 24-well plates. The number of seeding cells differs according to the cell proliferation rate.
Day 2: Target cells should be approximately 50%-70% confluent. For polybrene accessible cells, mix the culture medium with proper concentrations of polybrene. Replace the medium completely with 0.5 ml polybrene-containing medium. For polybrene sensitive cells, this step can be skipped.
Before infection, virus should be melted on ice gently and resuspended in culture medium. Remove the preceding medium and add lentivirus-containing medium with 1/2 volume of normal culture volume. Culture for 4 hours at 37℃, and supplement fresh medium to normal volume. The recommended medium volume of lentivirus infection is displayed in the following table 4.
| Culture dish type | Surface area | normal volume for cell culture | 1/2 volume for lentivirus infection |
| 96-well | 0.3 cm2 | 100 ul | 50 ul |
| 24-well | 2 cm2 | 500 ul | 250 ul |
| 12-well | 4 cm2 | 1 ml | 500 ul |
| 6-well | 10 cm2 | 2 ml | 1 ml |
Note:
1. Polybrene concentration
Though polybrene increases the efficiency of viral infection, it is toxic to some cell lines, and the sensitivity varies from different cell lines. For polybrene accessible cells We recommend the working concentration of polybrene as 6-8μg/ml.
2. Optimal MOI detection for cell infection
MOI (multiplicity of infection) refers to the number of infected viral particles per cell. For actively dividing cells such as HeLa or 293 cells, over 80% of the cells can express target genes with MOI of 1-3. For the non-dividing cells like primary cells with a low infection efficiency, we recommend testing a range of MOIs to determine the optimal MOI for infection and gene expression in target cell lines. The lentiviral MOIs of some common cell line are shown in the table 5.
| Cell line | MOI range | Auxiliary infection reagent polybrene (need/no) |
| K562 | 20~40 | Need |
| Jurkat | 50~80 | no |
| kasumi | 10~30 | no |
| NB4 | 50~80 | no |
| U937 | 20~40 | Need |
| THP-1 | 50~80 | Need |
| GBC-SD | 30~50 | no |
| H929 | 100~150 | no |
| H1299 | 1~3 | Need |
| 95D | 2~4 | Need |
| A549 | 20~40 | Need |
| SPC-A-1 | 100~150 | Need |
| 7402 | 10~15 | Need |
| Hep 3B | 10~30 | Need |
| Hep G2 | 10~30 | Need |
| SMMC-7721 | 10~30 | Need |
| Huh-7 | 10~30 | Need |
| Hela | 10~30 | Need |
| HOS | 20~40 | Need |
| Hep-2 | 10~30 | Need |
| HL-60 | >100 | Need |
| HT-29 | 10~30 | Need |
| PKO | 2~4 | Need |
| SW480 | 10~30 | Need |
| DLD-1 | 10~30 | Need |
| SK-OV-3 | 2~4 | Need |
| SHG-44 | 10~30 | Need |
| U251 | 1~3 | Need |
| U87 | 1~3 | Need |
| 293T | 1~3 | Need |
| HUVEC-2C | 10~30 | Need |
| PC-3 | 20~40 | Need |
| MDA-MB-231 | 10~30 | Need |
| MCF-7 | 20~40 | no |
| Tca8113 | 10~30 | Need |
| RPE | 10~30 | Need |
| AGS | 100~150 | Need |
| BGC-823 | 100~150 | Need |
| SGC-7901 | 10~30 | Need |
| MKN-28 | 20~40 | Need |
| MKN-45 | 20~40 | Need |
| BxPc-3 | 20~40 | Need |
| CFPAC-1 | 50~80 | Need |
| Panc-1 | 2~4 | Need |
| HEC-1-B | 2~4 | Need |
| NIH-3T3 | 20~40 | Need |
| Raw264.7 | 10~30 | no |
| CHO | 20~40 | Need |
| HSC-T6 | 10~30 | no |
| C6 | >100 | Need |
| NRK | 10~30 | Need |
Note: Influenced by cell source, algebra and cell state, the MOI values in different laboratories may be different to some extent. Data in this table are obtained on condition that the cell is in good state, and infection efficiency can reach 85-100% cells.
Day3: Refresh the culture medium 24 hours post infection.
Day4: Change to fresh medium with puromycin 48 hours post infection. The recommended concentration of puromycin ranges from 1 to 10μg/ml according to cell lines. Set the uninfected wild-type cells as control group and add equal volume and concentration of puromycin. Replace with fresh puromycin-containing medium every 2 or 3 days until the control group cells die out. Then choose one of the following steps according to experimental requirements.
1. Non-selecting monoclonal cells Passage the infected cells and select with puromycin constantly. Freeze the cell mixture in continuous three passages. Considering the heterogeneity, we recommend selecting monoclonal cell for a confirmed phenotype.
2. Selecting monoclonal cells Select at least five monoclonal cells after infection and puromycin selection, and propagate in puromycin-containing medium. Detection the expression of target genes using western blot or qPCR. Choose the stable cell line with proper expression level of target genes to passage three generations and freeze the stable cell line.
Notes for infection of special cell lines.
1. Suspension cells
We recommend using flat fillet centrifuging transfection to infect suspension cells or semi-suspension cells. Add virus suspension into cell culture dish, sealing tightly, and centrifuge at low speed of 200×g for 1 hour in the flat fillet centrifuge. Place cells in cell culture incubator after centrifuging transfection. If the flat fillet centrifuge is inaccessible, you can suspend the cells and transfer cells into centrifuge tubes, followed by low-speed centrifuge, and discard the most of supernatant. Add virus suspension into the tubes, resuspending cells, place it at room temperature for 15 min (no more than 30 min), and transfer the cells and virus suspension into plate to culture. Replace with fresh culture medium the next day.
2. Cells difficult to infect
For cells difficult to infect, like DC cells, we recommend repeated infections. Replace with fresh virus suspension 24 hours after the first infection. Repeated infections can increase the infection efficiency markedly.
3. Non-dividing primary cells
We recommend high-titer adenovirus to infect these cells like BMSC.
Detailed information of Lentivirus protocol can be seen in Lentivirus Protocol.
8. Lentivirus vector-Production Protocol, Guidelines And References
Lentivirus Production Protocol–Genemedi
1. Lentivirus plasmid construction
The gene of interest is cloned into one of the LTR/MCS-containing lentivirus vectors to generate pLV-GOI. The purity and RNA contaminants of viral plasmid should be taken into consideration.
2. Lentivirus packaging
The recombinant lentivirus viral plasmid pLV-GOI is co-transfected into the 293T with envelope expressing plasmid pMD2G and packaging plasmid pSPAX2, which together supply all the trans-acting factors required for lentivirus replication and packaging in the 293T cells.
3. Harvesting virus particles
Collect the supernatant containing lentivirus particles 48 hours and 72 hours post transfection, respectively. Replace fresh the culture medium after the collection of supernatant at 48h.
4. Virus purification
After collecting virus twice, discard the transfected 293T cells and filter the collected supernatant with 0.45μM filter membrane to an ultracentrifuge tube. Centrifuge at 72000g for 2 hours at 4℃. Then discard the supernatants and resuspend the lentivirus deposition with 500μl fresh medium and keep at -80℃ or in liquid nitrogen for long time storage.
5. Lentivirus titer detection
Determine lentivirus titer with fluorescent microscopy. Seed 293T 1×104 cells/well in a 96-well plate one day in advance and perform gradient dilution of the lentiviral particles to 1:10, 1:100, 1:1000, 1:10^4, 1:10^5, 1:10^6 in 100ul final volume in culture medium. Total 100ul viral particle mixture should be added to each well with at least 3 replicates per virus. Two days post infection, count the fluorescent positive cells using fluorescent microscopy and select the dilution factor with a proper fluorescent positive proportion (10%-30% positive cells/well). Count the triplicates and average the number of positive cells. Estimate the lentivirus titer using the following formulation: Viral titer (TU/ml) = number of fluorescent positive cells × 10 × dilution
6. Quality control of lentivirus
After lentivirus titer detection, the infection activity needs to be evaluated before animal experiment by infecting cells such as 293T, CHO to test the gene expression.
lentivirus – Guidelines
1.Salgado CD and JM Kilby. (2009). Retroviruses and other latent viruses: the deadliest of pathogens are not necessarily the best candidates for bioterrorism. J S C Med Assoc 105:104-6.
2.Piguet V, O Schwartz, S Le Gall and D Trono. (1999). The downregulation of CD4 and MHC-I by primate lentiviruses: a paradigm for the modulation of cell surface receptors. Immunol Rev 168:51-63.
3.Cockrell AS and T Kafri. (2007). Gene delivery by lentivirus vectors. Mol Biotechnol 36:184-204.
4.Vectors used in gene therapy clinical trials. The Journal of Gene Medicine Online Library. [Online] Updated Nov 2017.
5.Milone MC and U O’Doherty. (2018). Clinical use of lentiviral vectors. Leukemia 32:1529-1541.
6.Cavazzana-Calvo M, E Payen, O Negre, G Wang, K Hehir, F Fusil, J Down, M Denaro, T Brady, K Westerman, R Cavallesco, B Gillet-Legrand, L Caccavelli, R Sgarra, L Maouche-Chretien, F Bernaudin, R Girot, R Dorazio, GJ Mulder, A Polack, A Bank, J Soulier, J Larghero, N Kabbara, B Dalle, B Gourmel, G Socie, S Chretien, N Cartier, P Aubourg, A Fischer, K Cornetta, F Galacteros, Y Beuzard, E Gluckman, F Bushman, S Hacein-Bey-Abina and P Leboulch. (2010). Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature 467:318-22.
7.Cartier N, S Hacein-Bey-Abina, CC Bartholomae, G Veres, M Schmidt, I Kutschera, M Vidaud, U Abel, L Dal-Cortivo, L Caccavelli, N Mahlaoui, V Kiermer, D Mittelstaedt, C Bellesme, N Lahlou, F Lefrere, S Blanche, M Audit, E Payen, P Leboulch, B l’Homme, P Bougneres, C Von Kalle, A Fischer, M Cavazzana-Calvo and P Aubourg. (2009). Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 326:818-23.
8.Biffi A, E Montini, L Lorioli, M Cesani, F Fumagalli, T Plati, C Baldoli, S Martino, A Calabria, S Canale, F Benedicenti, G Vallanti, L Biasco, S Leo, N Kabbara, G Zanetti, WB Rizzo, NA Mehta, MP Cicalese, M Casiraghi, JJ Boelens, U Del Carro, DJ Dow, M Schmidt, A Assanelli, V Neduva, C Di Serio, E Stupka, J Gardner, C von Kalle, C Bordignon, F Ciceri, A Rovelli, MG Roncarolo, A Aiuti, M Sessa and L Naldini. (2013). Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science 341:1233158.
9.Sessa M, L Lorioli, F Fumagalli, S Acquati, D Redaelli, C Baldoli, S Canale, ID Lopez, F Morena, A Calabria, R Fiori, P Silvani, PM Rancoita, M Gabaldo, F Benedicenti, G Antonioli, A Assanelli, MP Cicalese, U Del Carro, MG Sora, S Martino, A Quattrini, E Montini, C Di Serio, F Ciceri, MG Roncarolo, A Aiuti, L Naldini and A Biffi. (2016). Lentiviral hemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: an ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet 388:476-87.
10.Aiuti A, L Biasco, S Scaramuzza, F Ferrua, MP Cicalese, C Baricordi, F Dionisio, A Calabria, S Giannelli, MC Castiello, M Bosticardo, C Evangelio, A Assanelli, M Casiraghi, S Di Nunzio, L Callegaro, C Benati, P Rizzardi, D Pellin, C Di Serio, M Schmidt, C Von Kalle, J Gardner, N Mehta, V Neduva, DJ Dow, A Galy, R Miniero, A Finocchi, A Metin, PP Banerjee, JS Orange, S Galimberti, MG Valsecchi, A Biffi, E Montini, A Villa, F Ciceri, MG Roncarolo and L Naldini. (2013). Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science 341:1233151.
11.Cullen BR. (1991). Human immunodeficiency virus as a prototypic complex retrovirus. J Virol 65:1053-6.
12.Frankel AD and JA Young. (1998). HIV-1: fifteen proteins and an RNA. Annu Rev Biochem 67:1-25.
13.Kingsman SM and AJ Kingsman. (1996). The regulation of human immunodeficiency virus type-1 gene expression. Eur J Biochem 240:491-507.
14.Purcell DF and MA Martin. (1993). Alternative splicing of human immunodeficiency virus type 1 mRNA modulates viral protein expression, replication, and infectivity. J Virol 67:6365-78.
15.Schwartz S, BK Felber, DM Benko, EM Fenyo and GN Pavlakis. (1990). Cloning and functional analysis of multiply spliced mRNA species of human immunodeficiency virus type 1. J Virol 64:2519-29.
16.Kim SY, R Byrn, J Groopman and D Baltimore. (1989). Temporal aspects of DNA and RNA synthesis during human immunodeficiency virus infection: evidence for differential gene expression. J Virol 63:3708-13.
17.Yasutsugu Suzuki and Youichi Suzuki (July 20th 2011). Gene Regulatable Lentiviral Vector System, Viral Gene Therapy Ke Xu, IntechOpen, DOI: 10.5772/18155.
18.Russell WC. (2009). Adenoviruses: update on structure and function. J Gen Virol 90:1-20.
19.Forsman A and RA Weiss. (2008). Why is HIV a pathogen? Trends Microbiol 16:555-60.
20.Graham AN. (1979). Contribution of the International Journal of Social Psychiatry. Int J Soc Psychiatry 25:238-9.
21.Naldini L, U Blomer, P Gallay, D Ory, R Mulligan, FH Gage, IM Verma and D Trono. (1996). In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272:263-7.
22.Gasmi M, J Glynn, MJ Jin, DJ Jolly, JK Yee and ST Chen. (1999). Requirements for efficient production and transduction of human immunodeficiency virus type 1-based vectors. J Virol 73:1828-34.
23.Kim VN, K Mitrophanous, SM Kingsman and AJ Kingsman. (1998). Minimal requirement for a lentivirus vector based on human immunodeficiency virus type 1. J Virol 72:811-6.
24.Zufferey R, D Nagy, RJ Mandel, L Naldini and D Trono. (1997). Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol 15:871-5.
25.Miyoshi H, U Blomer, M Takahashi, FH Gage and IM Verma. (1998). Development of a self-inactivating lentivirus vector. J Virol 72:8150-7.
26.Zufferey R, T Dull, RJ Mandel, A Bukovsky, D Quiroz, L Naldini and D Trono. (1998). Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol 72:9873-80.
27.Wagner R, M Graf, K Bieler, H Wolf, T Grunwald, P Foley and K Uberla. (2000). Rev-independent expression of synthetic gag-pol genes of human immunodeficiency virus type 1 and simian immunodeficiency virus: implications for the safety of lentiviral vectors. Hum Gene Ther 11:2403-13.




