Protocol of SARS-CoV-2 Pseudovirus (PSV)-Based Neutralization Assay For Vaccines, therapeutic antibodies, peptides and compounds against COVID-19
Diagnostic antibodies and antigens for Companion Animal disease testing
● Rabbit
Diagnostic antibodies and antigens for Swine disease testing
Diagnostic antibodies and antigens for Avian disease testing
Diagnostic antibodies and antigens for Multiple animal disease testing
Diagnostic antibodies and antigens for Ruminant disease testing
● Deer
Diagnostic antibodies and antigens for infectious and non-infectious Equine/Horse disease testing
SOCAIL MEDIA

SARS-CoV-2 (2019nCoV) pseudotype virus (pseudovirus, PSV) for COVID-19 related vaccines and neutralizing antibodies evaluation.
The outbreak of COVID-19, caused by SARS-CoV-2 (2019-nCoV), has been a global public health threat and caught the worldwide concern. Due to its high pathogenicity and infectivity1, live SARS-CoV-2 should be handled under biosafety level 3 (BSL-3) conditions. GeneMedi has developed SARS-CoV-2 pseudovirus production system, from which the SARS-CoV-2 pseudotyped virus can be handled in biosafety level 2 (BSL-2)2.
GeneMedi’s SARS-CoV-2 (2019nCoV) pseudotype virus (pseudovirus, PSV) based neutralization assay is a standard evaluation procedure for COVID-19 related vaccines and neutralizing antibodies potency evaluation. GeneMedi’s SARS-CoV-2 PSV is the core ingredient of diagnostics for neutralization serology after vaccinotherapy.
GeneMedi’s SARS-CoV-2 pseudotyped virus includes wildtype and the spike mutation variants (D614G, S943P, V367F, G476S, V483A, H49Y, Q239K, A831V, P1263L, D839Y/N/E: D839Y, D839N, D839E). The GeneMedi’s SARS-CoV2 PSV panel help for all-in-one vaccinotherapy evaluation.
Application-SARS-CoV-2(2019nCoV) Pseudotyped Virus Based Neutralization Assay3
In the Pseudovirus-Based Neutralization Assay (PBNA), the inhibition of viral entry into cells by neutralizing antibodies is correlated to the decreased levels of firefly luciferase signals in the ACE2 expression cells (hACE2-HEK293T, Cat. GM-SC-293T-hACE201) .
GeneMedi’s Pseudovirus Based Neutralization Assay (PBNA) is a conventional assay method that is suitable for High-Throughout Screening (HTS) without live virus engaged. The Pseudovirus Based Neutralization Assay can be used for evaluating:
1) Neutralizing antibodies (NAbs)3,4
2) Peptides blockers5,6 (peptide inhibitors) or protein7,8
3) Types of Vaccines (Immunized serum)9
4) Compounds targeting Spike induced cell-fusion10.
Protocol of SARS-CoV-2 Pseudovirus (PSV)-Based Neutralization Assay
Materials
1. SARS-CoV-2 Pseudovirus-RFP-fLuciferase (GM-2019nCoV-PSV01)
2. Efforter cell: Alternative
A. hACE2-HEK293T stable cell line (GM-SC-293T-hACE2-01)
B. Wildtype HEK293T cell line, hACE2 vector for transfection (GMV-V-2019nCoV-041)
Pseudotyped virus of SARS-CoV-2 Spike Mutation Variants (D614G, S943P, V367F, G476S, V483A, H49Y, Q239K, A831V, P1263L, D839Y/N/E:D839Y, D839N, D839E), P2-mutated Spike protein trimer variant (P2-mutant,S1/S2 cleavage site(furin cleavage sequence)-mutant, trimerization modified), Spike(S1+S2) – B.1.1.7 lineage, Spike(S1+S2)-N501Y mutation, Spike(S1+S2)-HV 69-70 Deletion mutation(ΔH69/ΔV70))
Cat No. | Description | Order
|
|---|---|---|
Spike S943P mutation SARS-CoV-2(2019nCoV) Pseudotyped virus |
||
SARS-CoV-2 Pseudotyped virus packaging and production |
||
Spike D614G mutation SARS-CoV-2(2019nCoV) Pseudotyped virus |
||
Spike V367F mutation SARS-CoV-2(2019nCoV) Pseudotyped virus |
||
Spike G476S mutation SARS-CoV-2(2019nCoV) Pseudotyped virus |
||
Spike V483A mutation SARS-CoV-2(2019nCoV) Pseudotyped virus |
||
Spike H49Y mutation SARS-CoV-2(2019nCoV) Pseudotyped virus |
||
Spike Q239K mutation SARS-CoV-2(2019nCoV) Pseudotyped virus |
||
Spike A831V mutation SARS-CoV-2(2019nCoV) Pseudotyped virus |
||
Spike P1263L mutation SARS-CoV-2(2019nCoV) Pseudotyped virus |
Protocol
If your efforter cell is hACE2-HEK293T stable cell line, please begin in Step 2.
1.Transfect HEK293T with hACE2-GFP vector (GMV-V-2019nCoV-041) 24hrs before planting the cell into 96-well.
2.Plant the hACE2-HEK293T into 96-well (5,000~10,000 per well) overnight before SARS-CoV-2 PSV infection.
3.Generation of 100ul PSV-Sample mixture:
| 100ul PSV-Sample mixture | Volume |
| GM-2019nCoV-PSV01* | 50ul or 5ul |
| Sample(NAbs, peptides, serum, etc) | flexible (According to your own products) |
| Total | add culture medium to 100ul |
| * For GM-2019nCoV-PSV01-1, add 50ul in recommendation (range from20ul~100ul). For GM-2019nCoV-PSV01-2, add 5ul in recommendation. (range from2ul~10ul). | |
Incubate PSV-Sample mixture for 1h at room temperature.
4.Remove the medium of efforter cell in 96-well, add 100ul PSV-Sample mixture to 96well cells for infection. 3wells for a group.
5.Fluorescence imaging (RFP) 72hrs after SARS-CoV-2 PSV infection. The firefly luciferase reporter is measured by following the Promega Luciferase Assay Reagent manual.
Tips
If your samples are serum
A standard curve should be generated using serially diluted Nabs (neutralizing antibodies) as a positive control.
If your samples are therapeutic antibodies or peptides candidates
Dilute the samples into concentration gradient for IC50 value evaluation.
GeneMedi SARS-CoV-2 Pseudovirus (PSV) Based Cell Entry
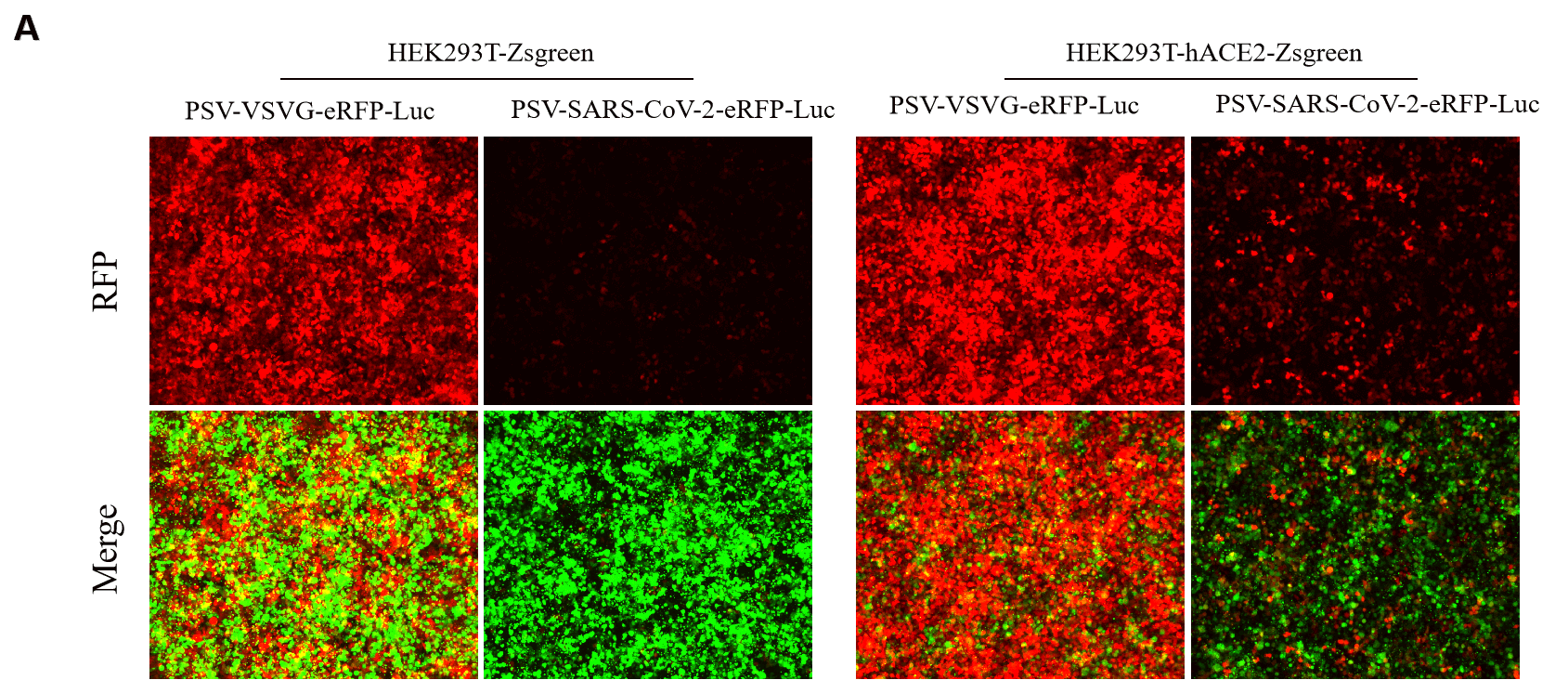
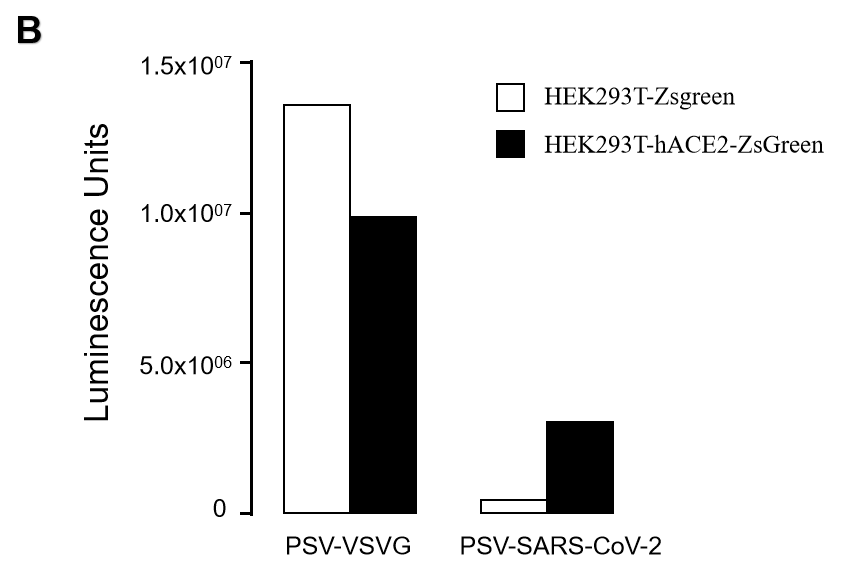
GeneMedi SARS-CoV-2 Pseudovirus (PSV) Based Cell Entry
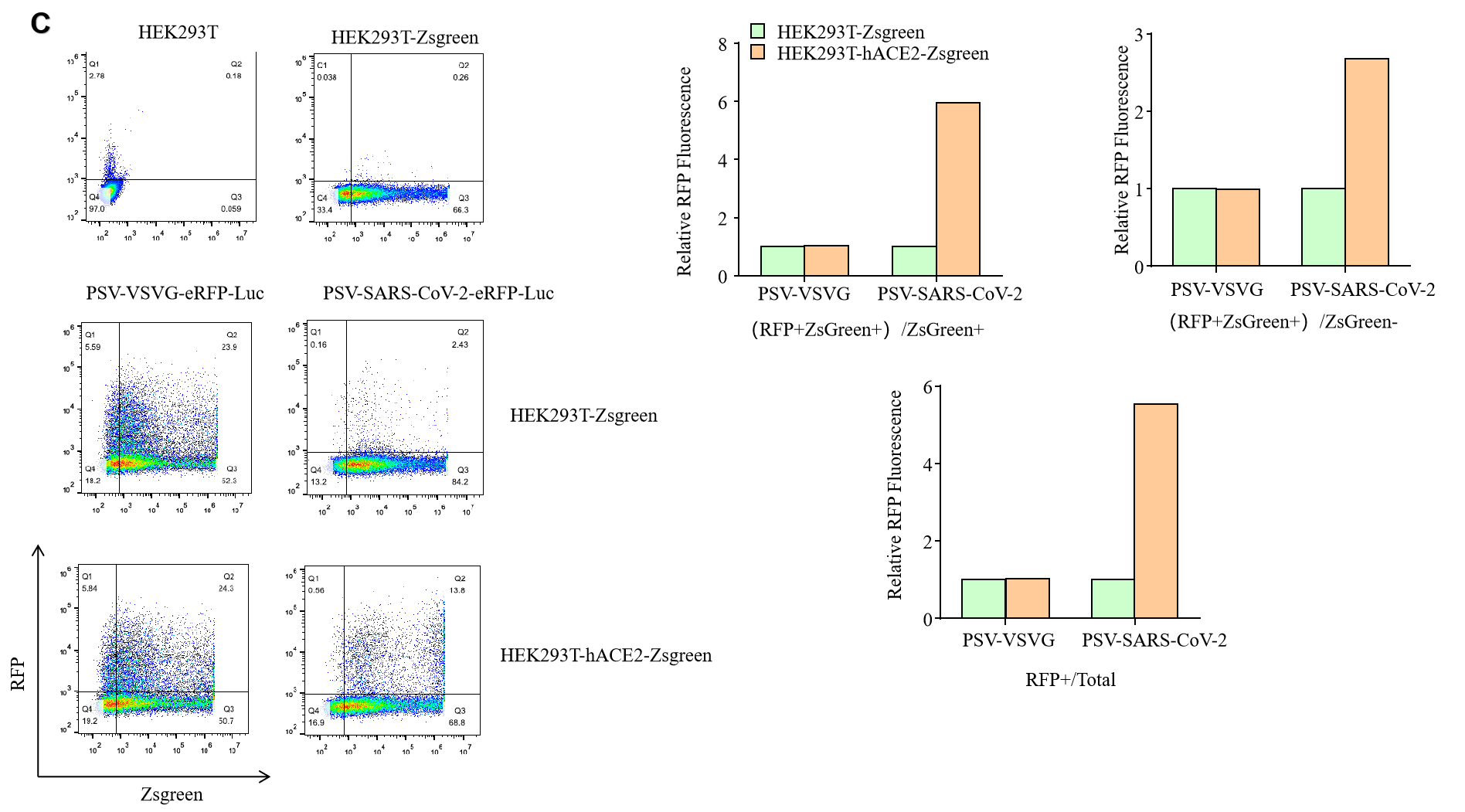
GeneMedi SARS-CoV-2 PSV-Luciferase (Cat.GM-2019nCoV-PSV01) is recombinant pseudotyped lentiviral particles containing SARS-CoV-2 spike protein to mimic SARS-CoV-2 (2019nCoV) cell infection.
GM-2019nCoV-PSV01 is a powerful tool for SARS-CoV-2 related vaccine efficacy evaluation, neutralizing antibodies, peptides blockers competitors neutralization assay, and tissue-specific infection determination.
References
1 XiaolongCai. An Insight of comparison between COVID-19 (2019-nCoV) and SARS-CoV in pathology and pathogenesis. Preprint, doi:10.31219/osf.io/hw34x (2020, paper of GeneMedi).
2 Jean K. Millet1, Tiffany Tang3, Lakshmi Nathan3, Javier A. Jaimes4, Hung-Lun Hsu3,5, & Susan Daniel3, G. R. W. Production of Pseudotyped Particles to Study Highly Pathogenic Coronaviruses in a Biosafety Level 2 Setting. J Vis Exp, doi:10.3791/59010 (2019).
3 Nie, J. et al. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg Microbes Infect 9, 680-686, doi:10.1080/22221751.2020.1743767 (2020).
4 Jiang, S., Hillyer, C. & Du, L. Neutralizing Antibodies against SARS-CoV-2 and Other Human Coronaviruses. Trends Immunol, doi:10.1016/j.it.2020.03.007 (2020).
5 Zhang, G., Pomplun, S., Loftis, A. R., Loas, A. & Pentelute, B. L. The first-in-class peptide binder to the SARS-CoV-2 spike protein. doi:10.1101/2020.03.19.999318 (2020).
6 Xia, S. et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res 30, 343-355, doi:10.1038/s41422-020-0305-x (2020).
7 Monteil, V. et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell, doi:10.1016/j.cell.2020.04.004 (2020).
8 Lei, C. et al. Neutralization of SARS-CoV-2 spike pseudotyped virus by recombinant ACE2-Ig. Nat Commun 11, 2070, doi:10.1038/s41467-020-16048-4 (2020).
9 HuajunBai, X., XiaolongCai. Vaccines And Advanced Vaccines: -A landscape for advanced vaccine technology against infectious disease ,COVID-19 and tumor. Preprint, doi:10.31219/osf.io/ypgx4 (2020).
10 Hoffmann, M. et al. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv, doi:10.1101/2020.01.31.929042 (2020).




