No products in the cart.
The sources of mycoplasma contamination in the laboratory are very challenging to completely control. As certain mycoplasma species are found on human skin, they can be introduced through poor aseptic technique. Additionally, they can come from contaminated supplements such as fetal bovine serum, and most importantly from other contaminated cell cultures. Once mycoplasma contaminates a culture, it can quickly spread to contaminate other areas of the lab. Strict adherence to good laboratory practices and routine testing for mycoplasma is highly recommended for successful mycoplasma removal.
Once mycoplasmas have been detected, the best solution to eliminate them and to prevent them from spreading is to discard the contaminated cell line. However, valuable cell lines that are too precious to be sacrificed can be salvaged by treatment with effective mycoplasma elimination reagents, including mycoplasma-selective antibiotics. These mycoplasma treatment reagent have been shown to eliminate mycoplasma and to restore cell behavior and responses within days or weeks after treatment. Till now, there are three classes of antibiotics that kill mycoplasma when used at relatively low concentrations: tetracyclines, macrolides and quinolones. Tetracyclines and macrolides block protein synthesis by interfering with ribosome translation, whereas quinolones inhibit replication of mycoplasma DNA.
Generally, there are four different approaches to the treatment of infected cell cultures with antibiotics: (1) the use of a single antibiotic compound (e.g., the fluoroquinolones), where basically the same procedure is employed for each antibiotic of that group; (2) the simultaneous application of two different antibiotics; (3) the use of a combination therapy applying two antimycoplasma agents subsequently in alternating cycles; and (4) the use of a membrane-active peptide to get rid of the majority of the mycoplasmas present in the medium and on the cells, followed by the application of an antibiotic to eliminate the remaining mycoplasmas inside and outside the cells. Based these idea, many mycoplasma removal kits comprised of different mycoplasma removal reagents are currently on the market.
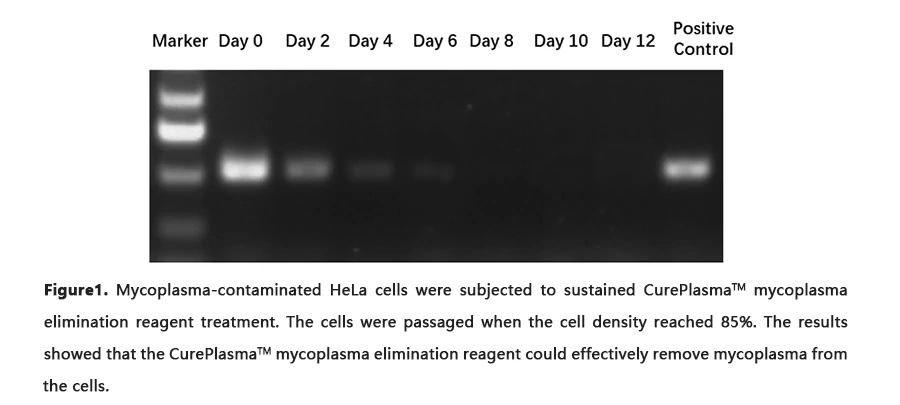
CurePlasma is a single ready-to-use mycoplasma elimination reagent that can be added directly to the culture medium. The two antibiotics in CurePlasma have distinct mechanisms: one blocks protein synthesis and the other stops DNA replication. Therefore, CurePlasma is more effective at removing mycoplasma and prevents the generation of resistant strains. Moreover, in contrast to other mycoplasma removal reagent, CurePlasma is active against free as well as intracellular forms of mycoplasma. It ensures that, following mycoplasma treatment, cell cultures do not become re-infected by mycoplasma that had previously been released from the intracellular compartments of infected cells. To date, no consistent or permanent alterations that affect eukaryotic cells during or after CurePlasma treatment have been reported.