No products in the cart.
Enzyme-Linked Immunosorbent Assays (ELISA) – strategies used in diagnosis of animal infectious disease for animal health
Diagnostic antibodies and antigens for Companion Animal disease testing
● Rabbit
Diagnostic antibodies and antigens for Swine disease testing
Diagnostic antibodies and antigens for Avian disease testing
Diagnostic antibodies and antigens for Multiple animal disease testing
Diagnostic antibodies and antigens for Ruminant disease testing
● Deer
Diagnostic antibodies and antigens for infectious and non-infectious Equine/Horse disease testing
SOCAIL MEDIA

Abstract - Animal health diagnosis
Animal infectious diseases pose a continuing threat to animal health, food safety, national economy, and the environment. Zoonotic infections, also named as zoonoses, involve veterinary pathogens that are sustained in animal populations but can be transmitted to and cause disease in humans. In the event of veterinary outbreaks, it is essential to make rapid and accurate diagnosis to control and prevent the spread of diseases. Here we discuss different diagnostic methods available to identify animal diseases and zoonotic infections. Efficient diagnosis strategies are critical for controlling and eliminating animal diseases and zoonoses, further protecting and improving animal health, quality, and productivity.
Enzyme-Linked Immunosorbent Assays (ELISA) - strategies used in diagnosis of animal infectious disease for animal health
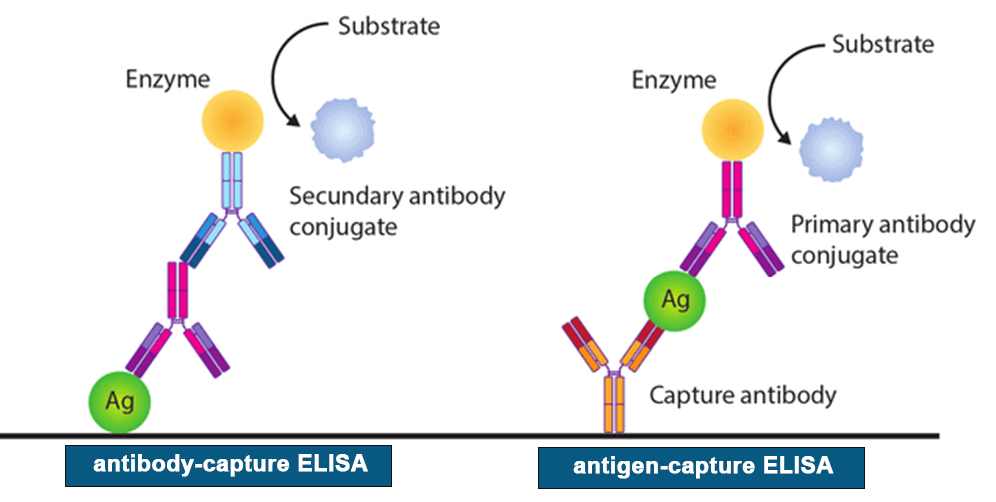
Enzyme-linked immunosorbent assays (ELISAs) incorporate the sensitivity of simple enzyme assays with the specificity of antibodies, by employing antigens or antibodies coupled to an easily-assayed enzyme. As such ELISA is much more rapid method than immunoblotting to detect specific viral protein from a cell, tissue, organ, or body fluid. There are two main variations of ELISAs: antigen-capture ELISA (detecting viral proteins), involve attachment of a capture antibody to a solid matrix for the viral protein of interest, while antibody-capture ELISA measures the specific antibody level in a sample, by coating viral antigen protein on a solid surface.
There are two principles based on antigen-capture and antibody-capture ELISAs. In a general, ELISAs are considered a highly sensitive method that can detect a fairly low number of proteins at the range of picomolar to nanomolar range (10-12 to 10-9 moles per liter). ELISA method was found useful as a diagnostic tool to detect influenza viral antigen much quicker than other conventional virus detection methods [7]. In another previous study, comparison of ELISA, with conventional methods has demonstrated ELISA superiority for the rapid detection and identification of influenza A virus [8]. A simplified and standardized neutralization enzyme immunoassay (Nt-EIA) was developed to detect measles virus growth in Vero cells and to quantify measles neutralizing antibody [9].
Newer EIA formats for hepatitis C virus diagnostics have been constantly evaluated [10, 11]. As such ELISAs are being used for plethora of application both in experimental and diagnostic virology including dengue, and influenza [12-14]. On the other hand, although rapid than traditional plaque assays or TCID50, ELISA assays sometimes could be quite expensive, due to the cost of reagents used. Unfortunately, sometimes required antibodies may not be commercially developed as well. In contrast, attempts to develop antibodies in-house may be quite expensive. Additional variability may also be introduced due to high background signals generated by non-specific binding, or cross-reactivity with non-viral protein targets.
Summary
In the recent years, importance of animal disease and their public health effects have been well recognized worldwide. Animal disease, more significantly, zoonotic disease cause human mortality and morbidity, and also affect livestock’s production, decrease availability of food and create barriers for international trade. Rapid diagnosis is critical for the implementation of efficient control strategies against animal disease and zoonotic disease. Understanding animal disease infection dynamics and collecting appropriate specimens at the appropriate time window are also important to obtain reliable diagnostic results. A number of virological and serological methods have been developed and used for animal disease diagnostic testing. RT-PCR is the method of common choice for the detection of animal disease; IHC combined with hematoxylin and eosin staining has also been commonly used to examine histopathological lesions caused by animal disease. Success rate of virus isolation in cell cultures has been low. Serological assays can provide information about previous exposure to animal disease and also determine antibody responses to infection or vaccination when vaccines are available. Rolling out serological test would be an effective strategy to determine the percentage of the population that is immune and have shown no symptoms for the animal disease. Thereby, determining the exact magnitude of the outbreak and enabling governments to assess containment strategies to slow down the spread. The major drawbacks with these immunoassays are their accuracy and sensitivity of the test results. Therefore, there needs to be extensive research and testing done to develop new cost-effective methods to quickly and easily determine animal disease infection. Whereas, any such emerging approach must be carefully evaluated for its efficiency, accuracy, and linear range. The FDA approval and evaluation of each diagnostic technique is necessary before it can be used in practice.
Full product list: Ruminants, Pet, Swine, Equine, Avian, Fish, Multiple Species
Validated animal health diagnostic antibodies pairs and antigens for animal infectious diseases diagnostic testing in ELISA, Lateral flow immunoassay (LFIA) and other immunoassays.
GeneMedi offers paired antibodies and antigens for Animal Health Diagnostic testing including most of the infectious disease in different animals:Avian(birds), fish, pets(cat, dog, rabbit), pig, ruminants(cow, goat, sheep, ox, cattle, bull) and so on(please see below).
All our animal health diagnostic antibodies and antigens for antimals infectious diseases test are suitable for in functional sandiwich ELISA, and other immunoassays in diagnostics. The antibodies can act as a capture antibody and detection antibody. The antigens can be used for antibodies rapid test of infectious disease.
Reference:
1. Ryu, S., et al., One Health Perspectives on Emerging Public Health Threats. J Prev Med Public Health, 2017. 50(6): p. 411-414.
2. World Health Organization (WHO). Zoonoses. Accessed October 3, 2018.; Available from: http://www.who.int/zoonoses.
3. Leslie MJ, M.J. Surveillance for zoonotic diseases. BLUKO97- Mikanatha 2007; Available from: http://courses.washington.edu/zepi526/Papers08/Rabies%20chapter.pdf.
4. Available from: https://www.doctorsgate.com/en/what-are-the-current-diagnostic-tests-for-covid-19/.
5. Anylab. Available from: www.zetbio.com.
6. Li, H., et al., A new and rapid approach for detecting COVID-19 based on S1 protein fragments. Clin Transl Med, 2020. 10(2): p. e90.
7. Khanna, M., et al., Evaluation of influenza virus detection by direct enzyme immunoassay (EIA) and conventional methods in asthmatic patients. J Commun Dis, 2001. 33(3): p. 163-9.
8. Waner, J.L., et al., Comparison of Directigen FLU-A with viral isolation and direct immunofluorescence for the rapid detection and identification of influenza A virus. J Clin Microbiol, 1991. 29(3): p. 479-82.
9. Cameron, J.D., A.P. Skubitz, and L.T. Furcht, Type IV collagen and corneal epithelial adhesion and migration. Effects of type IV collagen fragments and synthetic peptides on rabbit corneal epithelial cell adhesion and migration in vitro. Invest Ophthalmol Vis Sci, 1991. 32(10): p. 2766-73.
10. Kim, M.H., S.Y. Kang, and W.I. Lee, Evaluation of a new rapid test kit to detect hepatitis C virus infection. J Virol Methods, 2013. 193(2): p. 379-82.
11. Niu, X., et al., [Establishment of the evaluation reference system for domestic anti-hepatitis C virus diagnostic enzyme immunoassay kits]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi, 2013. 29(7): p. 761-4.
12. Filice, G., et al., Sensitivity and specificity of anti-HIV ELISA employing recombinant (p24, p66, gp120) and synthetic (gp41) viral antigenic peptides. Microbiologica, 1991. 14(3): p. 185-94.
13. de Boer, G.F., W. Back, and A.D. Osterhaus, An ELISA for detection of antibodies against influenza A nucleoprotein in humans and various animal species. Arch Virol, 1990. 115(1-2): p. 47-61.
14. Cuzzubbo, A.J., et al., Comparison of PanBio dengue duo enzyme-linked immunosorbent assay (ELISA) and MRL dengue fever virus immunoglobulin M capture ELISA for diagnosis of dengue virus infections in Southeast Asia. Clin Diagn Lab Immunol, 1999. 6(5): p. 705-12.
15. Maria-C-Jimenez-Martinez. Available from:
https://www.researchgate.net/profile/Maria-C-Jimenez-Martinez/publication/320265684/figure/fig3/AS:547016444395520@1507430291554/ELISA-assays-Direct-ELISA-mostly-used-for-antigen-detection-Indirect-ELISA-mainly-used.png.
16. sciencephoto. Available from: https://www.sciencephoto.com/media/90184/view.
17. Uyeki, T.M., et al., Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenzaa. Clin Infect Dis, 2019. 68(6): p. e1-e47.
18. Goldsmith, C. Hantavirus Life Cycle and Infection Process. Available from: https://www.hantasite.com/2017/03/hantavirus-life-cycle-and-infection.html.
19. METHODS, M. 2013; 3:207
20. biotech, G.; Available from: https://www.globalbiotechinsights.com/articles/20247/the-worldwide-test-for-covid-19.